In my previous article, I questioned how our simulation community of experts can contribute in this pandemic time? I am coming back to present examples of contributions. While the general topic of this article is outside the typical scope of Electronics Cooling Magazine, it does illustrate how analysis methods that are discussed here, such as computational fluid dynamics, and technologies that are part of our industry, such as lighting, can be applied to address a problem that is both significant and timely to the entire world.
Facts and figures
Since April 2020, already 3.3 million people have died of COVID-19 as recorded by Johns Hopkins university [1]. Worldwide, entities including research institutes, public health organizations, and national governments have been working for months to reduce the impact of the pandemic. Many measures, such as social distancing, using personal protective equipment, improving the safety of public spaces through proper ventilation, and developing vaccinations to ultimately achieve herd immunity have been implemented. This article describes a less well recognized, but important tool available for improving safety: upper room ultraviolet germicidal irradiation (UVGI) disinfection. On February 25th, 2021, the Center for Global Health Delivery, Advance Access & Delivery, the Belfer Center’s Middle East Initiative at the Harvard Kennedy School, and Harvard Global Health Institute, hosted a webinar featuring a panel of engineers and infectious disease experts to talk about the safety and evidence base supporting the use of UR-GUV [2], [3].
This article discusses the concept of using ultraviolet light (UV-C) for disinfecting upper air, i.e., creating a disinfection zone near the ceiling of a room to avoid direct exposure of people to any dangerous UV radiation. It begins by describing the basics of UV, the airborne transmission route of SARS-CoV-2 and then explains how UV-C can disinfect the air. Finally, an example of how multi-domain simulation is used to assess the challenges, as well as the advantages of this approach, is presented.
UV-C has a long history and track record in effective disinfection
UV-C is a well-established method for disinfection. UV-C light is a category of ultraviolet light with wavelengths between 100-280 nanometres (nm) and is the most effective UV light for disinfection. It has been applied ever since it was discovered to be an effective tool in preventing the spread of contagious diseases by disinfecting water, surfaces and air in very short time periods. UV-C light inactivates viruses and microorganisms such as bacteria, molds, spores, fungi and yeasts by destroying their DNA or RNA. It is generated by lamps, using well-known technology. Additionally, it is sustainable and more environmentally friendly than several other disinfecting means, e.g. chemical.
The first observation that microorganisms respond to light was published by Ludwig Karl Schmarda in 1845 [4]. The germicidal effect of ultraviolet light was discovered by Downes and Blunt in 1877 [5]. When they placed solution-filled test tubes outdoors, they discovered that sunlight could kill and inhibit the development of pathogenic bacteria. In 1935, Wells [6] demonstrated that UV-C, which had been used to kill microorganisms on surfaces and in liquids, could also be used to kill airborne infectious organisms. In the late 1950s and early 1960s, Riley et al. [7][8] conducted a series of animal experiments that conclusively showed that intense ultraviolet germicidal irradiation (UVGI) in air ducts inactivates virulent M. tuberculosis in droplet nuclei. In 1975, Riley et al. aerosolized Bacillus Calmette-Guérin (BCG) into a test room and measured its inactivation with and without upper-room UV-C. They found a sixfold increase in the inactivation rate in the rooms that had upper-room UV-C installed [9]. In the late 1980s, there was a renewed interest in UV-C due to the unexpected rise in tuberculosis (TB) and the emergence of multiple drug-resistant strains. In 2009, Escombe et al., [10] published a clinical trial using upper-room UV-C as an effective, low-cost intervention to prevent TB transmission in high-risk clinical settings. Mphaphlele’s paper [11] in 2015 showed that upper room germicidal UV air disinfection with air mixing was highly effective in reducing tuberculosis transmission under hospital conditions and included improved evidence-based dosing guidelines.
Through the years, UV-C light has been proven to inactivate, without exception, all micro-organisms and viruses against which it has been tested, including, among others, those causing tuberculosis, influenza and SARS-CoV-1 [12].
The scientific basics of UV-C germicidal efficiency are well understood
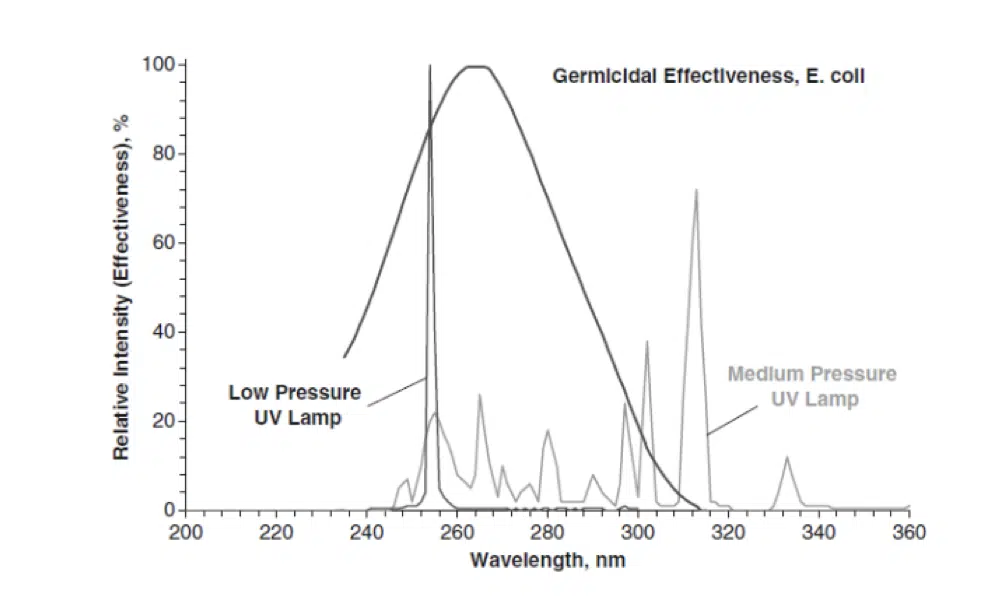
Ultraviolet Germicidal Irradiation is electromagnetic radiation that destroys the ability of pathogens to reproduce by causing photochemical changes in nucleic acids. The wavelengths in the UV-C range are particularly damaging to pathogens because they are absorbed by proteins, RNA, and DNA. The germicidal effectiveness of UV is typically represented by the graph shown in Figure 1 and originally published by Gates in 1930 [13]. The germicidal action spectrum with peak effectiveness at 265 nm coincidentally overlaps with the 253.7nm peak of low-pressure mercury UV lamps. Although the germicidal effectiveness can vary between species, the curve for E. coli is very typical for common pathogens.
Figure 1. Germicidal efficiency of UV wavelengths, comparing High (or medium) and low-pressure UV lamps with germicidal effectiveness for E. coli. [18]
Upper-air UV-C systems can inhibit the likely airborne transmission route of SARS-CoV-2
Several studies indicate that airborne transmission is a significant factor in the spread of the SARS-CoV-2 virus and of other viruses that cause diseases like SARS, MERS, and influenza [14][15][16]. On 30 April 2021, the WHO updated its Q&A page with a warning of airborne transmission of SARS-CoV-2 to say “The virus can spread from an infected person’s mouth or nose in small liquid particles when they cough, sneeze, speak, sing or breathe. These particles range from larger respiratory droplets to smaller aerosols.” [17]. Airborne transmission is further backed up by scientific publications describing, for example, the Skagit Valley chorale superspreading event [18], [19], [20], [21].
Natural air flows resulting from movement, temperature changes and recirculating air-conditioning in indoor spaces contribute to the rapid spread of viruses like SARS-CoV-2. This is an obvious challenge in battling the virus, as air cannot be easily contained. However, the risks can be mitigated by applying UV-C light to reduce the virus concentration in the air using in-duct and upper-air disinfection systems, while at the same time preventing human exposure to UV-C irradiation. Indeed, both solutions leverage air flow models to provide the right UV-C intensities to achieve effective disinfection.
Recently, both the WHO and CDC recommended [22][23] the use of upper-room UVGI systems as a supplemental air-cleaning measure to reduce the transmission of airborne pathogens in public buildings, hospitals, military housings, and classrooms.
The metrics behind UV-C effectiveness assessment
The first theoretical models describing the UV disinfection process and related decay models were described by Hiatt in 1964 [24]. The UV-C effectiveness is typically expressed as the fraction of pathogens killed or inactivated by the UV-C irradiation compared to the situation without UV-C irradiation [25]. How many pathogens are inactivated as a result of UV exposure depends on the received UV dose D and the UV susceptibility k (the ‘k-value’) of the pathogen. Theoretically, the higher the k-value for a target pathogen, the more quickly the pathogen will be killed or inactivated by UV-C irradiation:
NUV / N0 = e -kD
where
N0 is the number of surviving microorganisms with no UVGI exposure,
NUV is the number of surviving microorganisms follow- ing UV exposure, and
D is the UVGI dose
As indicated by this formula, pathogens exposed to UV-C irradiation typically decrease exponentially. Another common metric that is used to quantify the effectiveness of UV-C irradiation is the UV-C dose required to inactivate 90% of the initial population: D90. The corresponding D90 values for a wide range of pathogens can be found in UVGI handbooks (e.g., Kowalski [25]) but also in standards like ISO 15714 [26]
Log Reduction Value (LRV) (sometimes called Log reduction factor)
Log reduction is commonly used to express the relative number of pathogens inactivated by disinfection. A 1-log reduction corresponds to inactivating 90 percent of a target pathogen, or equivalently a reduction of the pathogens count by a factor 10. Respectively, a 2-log and 3-log reduction correspond to inactivating 99% and 99,9%, or equivalently a reduction by a factor 100, 1000.
Log reduction = log10 (N0 / NUV) (Equation 1)
UV dose and susceptibility
Effective disinfection systems achieve the desired log reduction factor by using the adequate level of pathogen-specific UV-C dose.
Every pathogen, based on its biological make-up, has a unique spectral susceptibility, which is characterized with the coefficient k in the previous equation. Scientific studies have shown so far that all tested pathogens are inactivated by UV-C, and SARS-CoV-2, the virus that causes COVID-19, is no exception [27]. However, the exact UV-C susceptibility of SARS-CoV-2 has not been measured and reported yet. Nevertheless, data on other (corona)viruses is available.
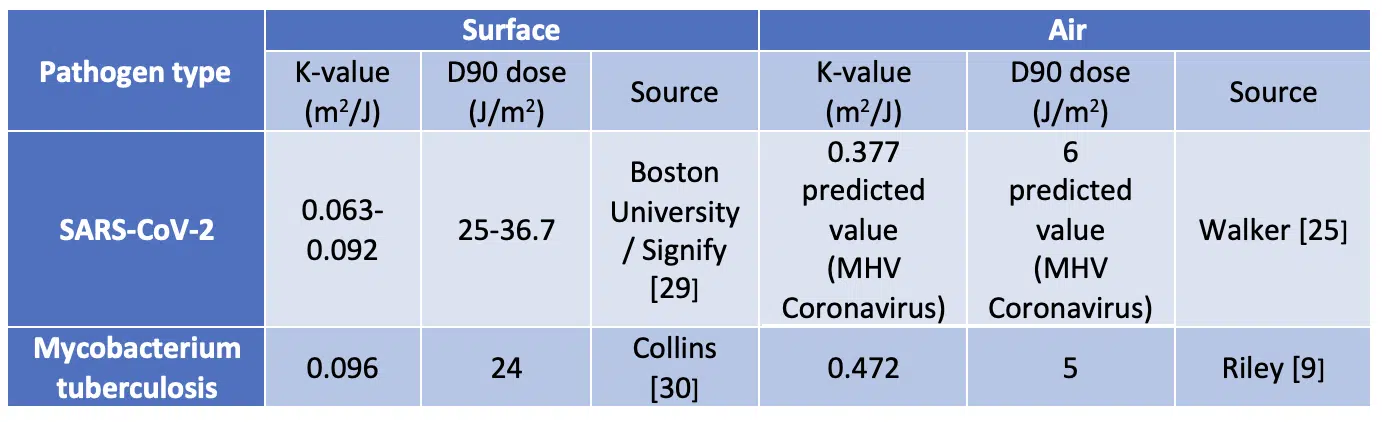
One of the first quantitative measurements of the effect of UVGI on one of the coronaviruses was performed by Walker and Ko [20] in 2007. They performed experiments on coronavirus aerosols in a single pass test rig. They measured a k-value of 0.377 m2/J for the Murine (Mouse) Hepatitis Virus (MHV) coronavirus.
An overview of the key experimental UV-C air and surface disinfection at 254nm studies is shown in Table 1.
Table 1. Examples of measured characteristics of pathogens and responses to UV-C
The relatively high k-value measured for the MHV Coronavirus suggests that UV-C disinfection may be an effective tool for inactivating the coronaviruses that cause diseases such as SARS, MERS as well as COVID-19. Beggs et al. [31] made an analysis of this data and other data available in literature to predict a ‘most likely range for k-factor of 0.377-0.590 J/m2 for SARS-CoV-2. This overview provides evidence that coronaviruses are as susceptible to UV-C disinfection when aerosolised as M. tuberculosis. In general, aerosolized viruses appear to be more vulnerable to UV damage than those suspended in a liquid or remaining on surfaces. Therefore, ASHRAE (The American Society of Heating, Refrigerating and Air-Conditioning Engineers) are working on UVGI UR Guidelines [32].
Modeling upper air disinfection with UV-C luminaire
When designing upper room disinfection systems based on UV-C, the key requirements are ensuring that people in the room are safe and the air is adequately disinfected. The first requirement is strongly dependent on the wavelength chosen for UV-C disinfection and the design of the luminaire itself. The second requirement depends on the dose received by the pathogens, which is determined by the actual air flow in the room in combination with the irradiance distribution in the UV-C disinfection zone. We will now focus on simulation of the latter.
Three elements are included in the simulation: the airflow in the room, the bio-aerosols released by a sick patient, and the irradiance from the UV-C luminaire.
The respiratory particles released by a sick patient normally can be categorized as being in one of three states. Large droplets often only travel short distances before falling to the floor. Depending on the conditions, such as temperature and humidity, smaller particle may either evaporate or remain as aerosols and travel further. These are of the greatest interest because they can stay in the air for longer times and potentially contaminate people. For the sake of simplicity in this present paper, only aerosols which mix with room air are modelled.
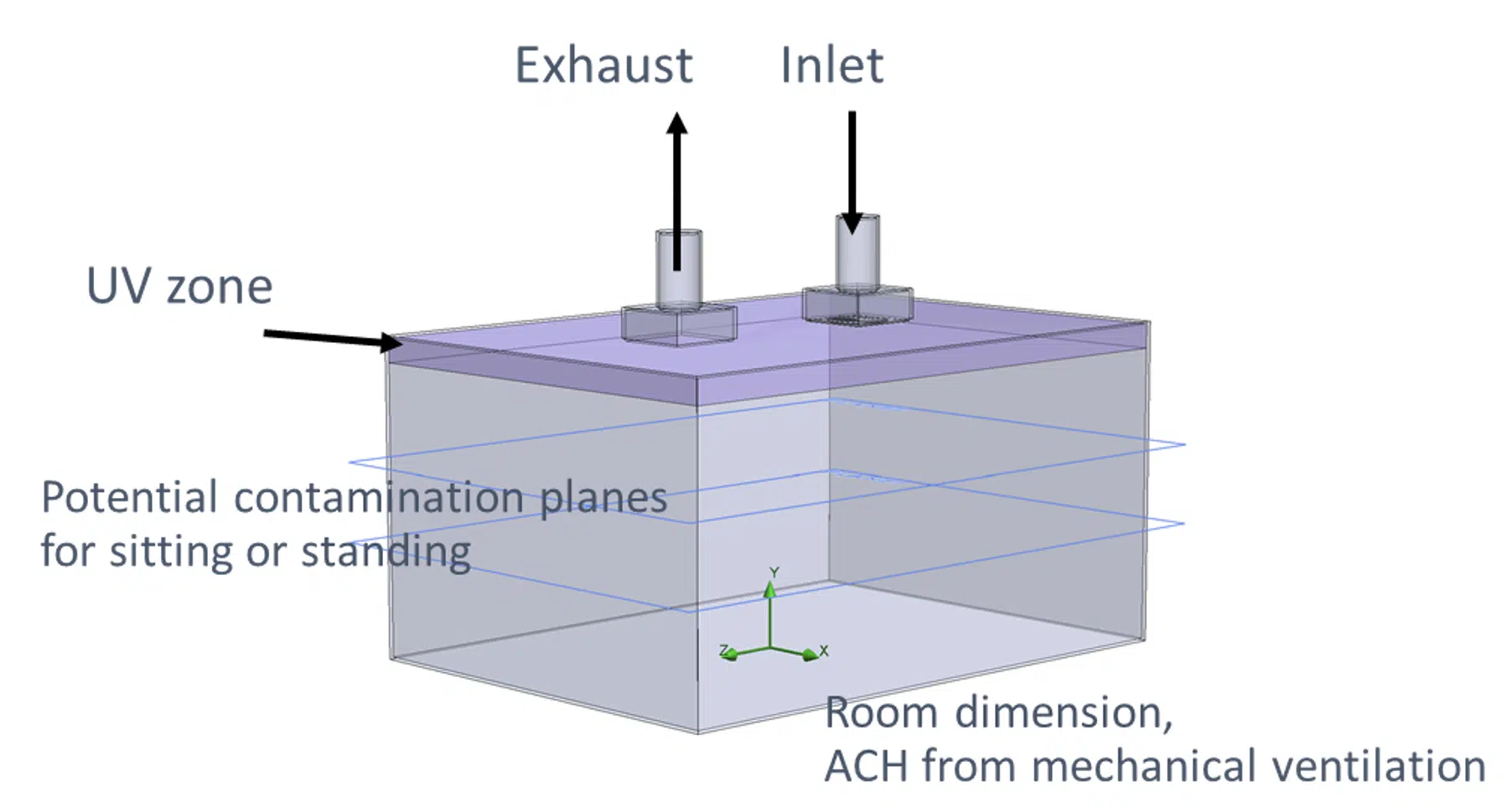
A 3D virtual model of a room with mechanical ventilation can be described as follows. It contains the room dimensions, the number of air changes per hour (ACH), determined by the ventilation (HVAC) system, the UV-C disinfection zone, which is dependent on the number of luminaires and their location, the person zone (where people are standing or sitting), the released contaminated aerosol, and the type of contaminant e.g., SARS-CoV-2. A schematic illustration of such a room is shown in Figure 2).
Figure 2. Example of simplified room model with mechanical ceiling ventilation
Numerous commercially available simulation tools are available for this type of modeling. This example used a tool that is capable of combining all of the above mentioned critical elements [32].
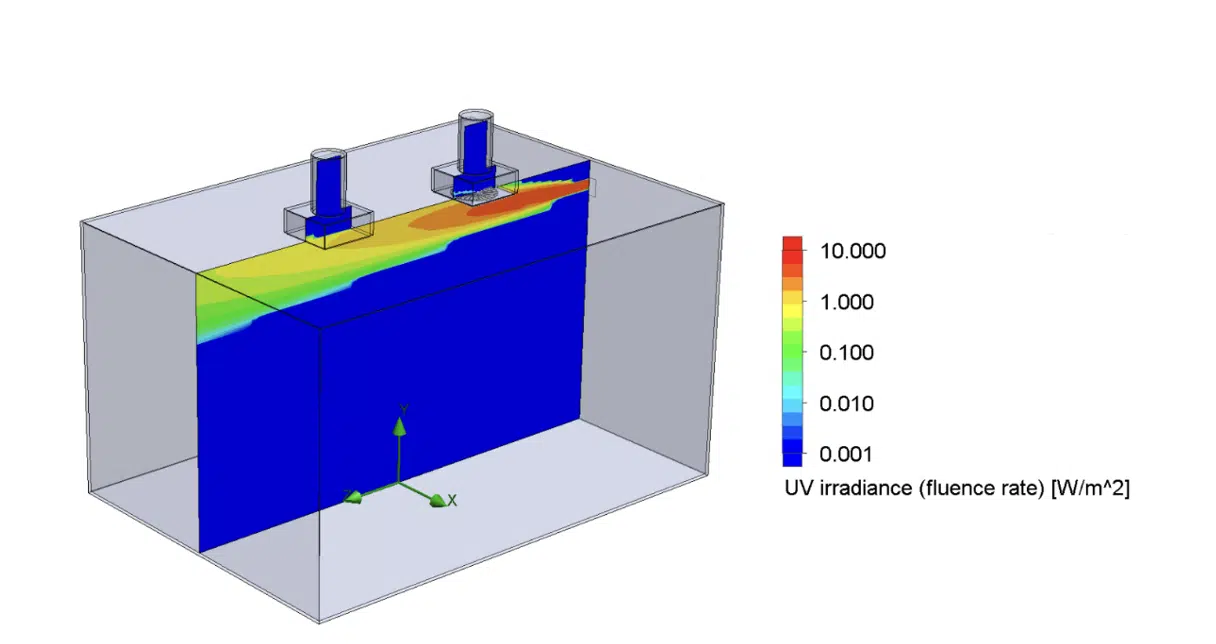
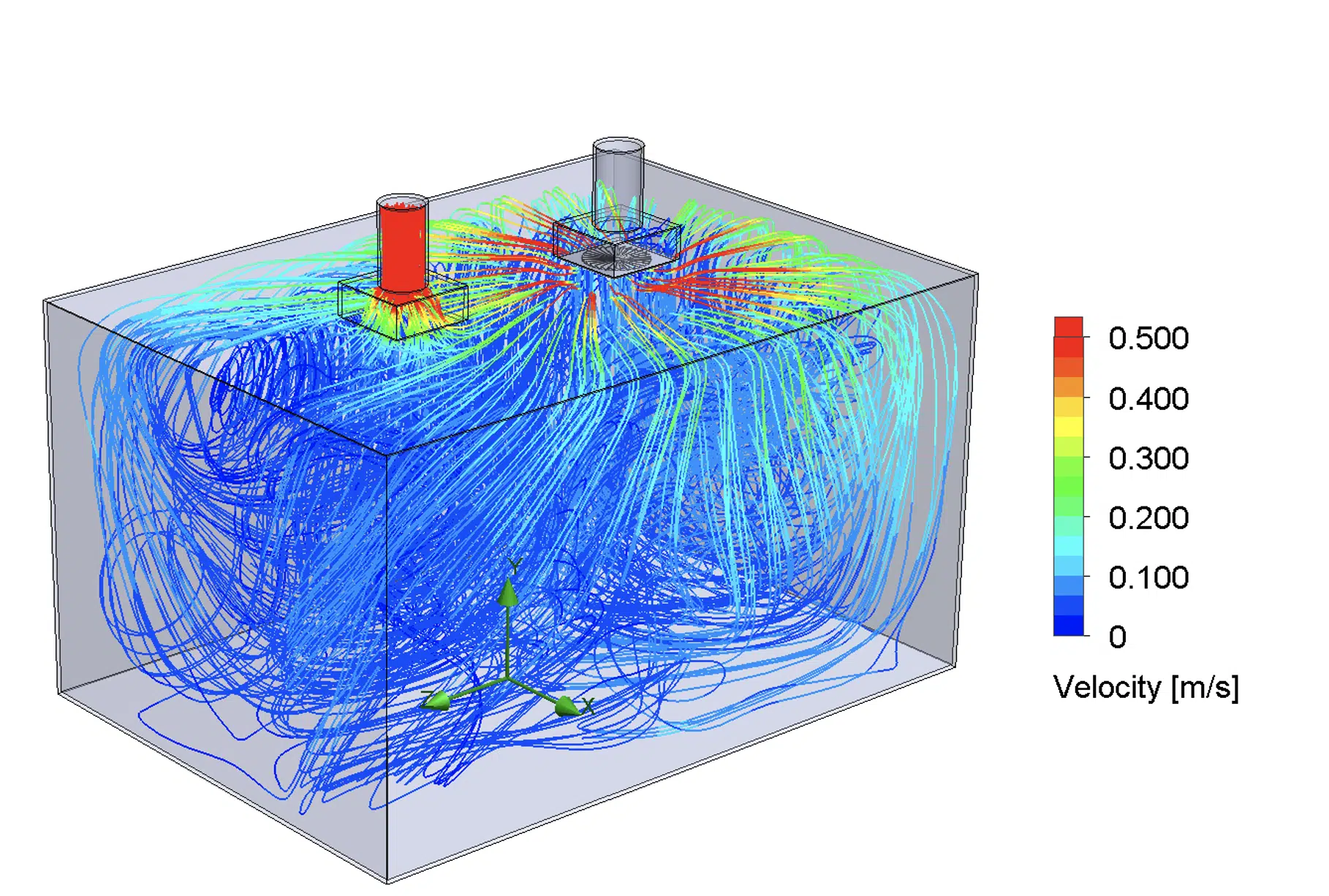
Figure 3 and Figure 4 show examples of irradiance and airflow distribution in the modeled room.
Figure 3. Example of UV irradiance for a UV source located on the back wall near the ceiling
Figure 4. Example of airflow simulation + particle trajectory
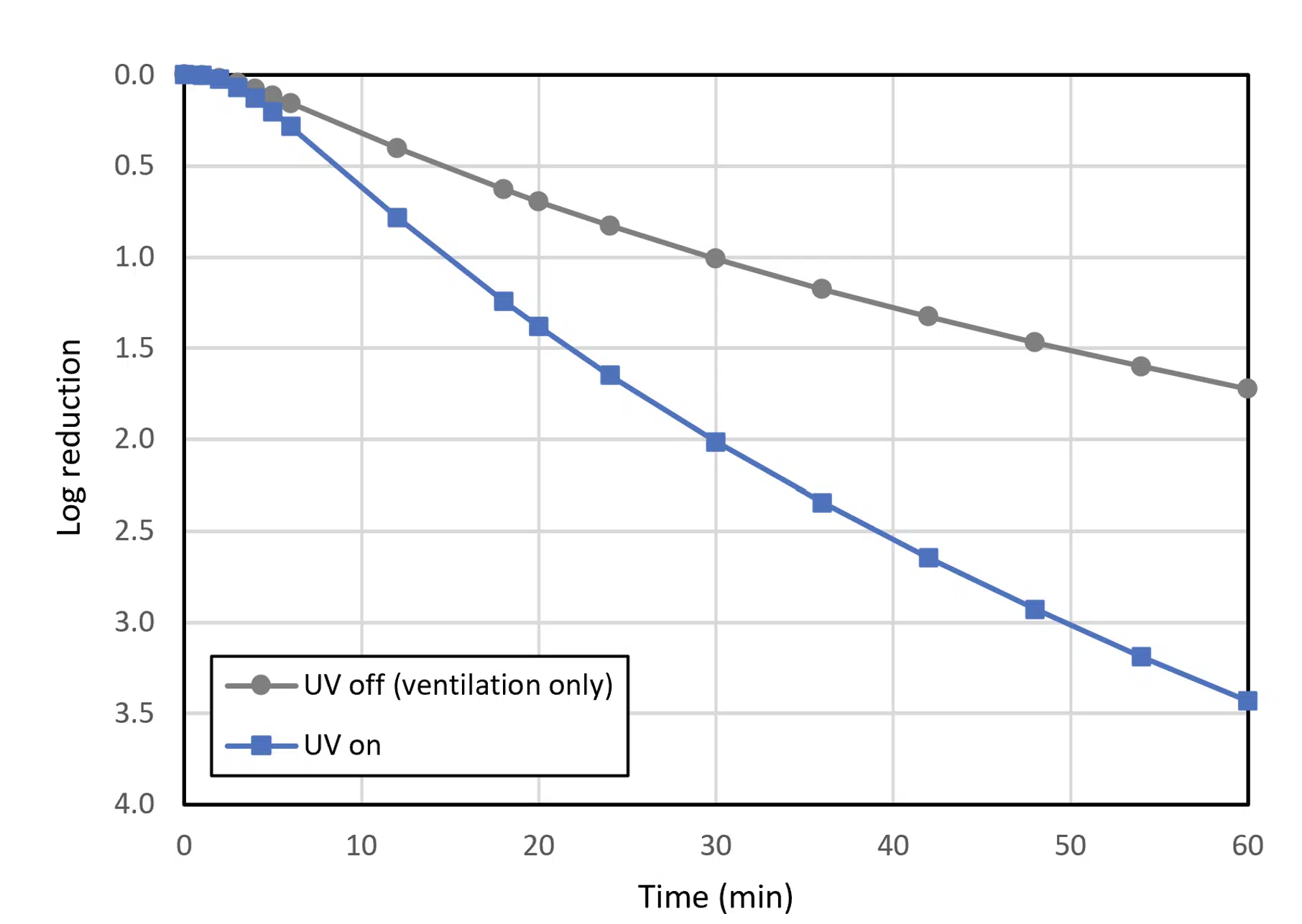
Figure 5. Log reduction of virus @ACH
In the air flow simulation particles were tracked over time. From the location history of the particles and the UV irradiance in the room, the UV dose accumulated by the particles is determined. Additionally, it is checked whether a particle is still in the room at a given time. This information can then be used to determine the remaining concentration of active pathogens in the room using equation (1). Alternatively, it can be checked how many particles have reached a certain threshold dose, such as D90.
Expressed in log reduction, Figure 5 shows a typical output for both cases with and without UV-C disinfection. In this particular example, the plot shows for instance that after 30 minutes, using UV dose together with ventilation achieves a 2-log reduction while with solely ventilation, only a 1-log reduction is achieved.
Advantages of using analysis methods such as computational fluid dynamics
In summary, considering the difficulty and related risks of doing experiments with an active lethal virus, being able to provide 3D simulations of the target applications presents a substantial advantage to quantify and further optimize the efficacy of UV-C disinfection systems. It also provides objective metrics to compare the germicidal efficacy of different engineering air disinfection technologies. Thereby, the air disinfection efficacy can for example be expressed using the log reduction value as a function of time. Optimizing upper room UV C systems using analysis methods, such as computational fluid dynamics, will be essential in the fight against present and future airborne diseases, like SARS-CoV-2.
References
1. Johns Hopkins: COVID-19 Map – Johns Hopkins Coronavirus Resource Center (jhu.edu) (Accessed May 14th, 2021)
2. Harvard medical school – Center for global health delivery: https://ghdcenter.hms.harvard.edu/keeping-public-spaces-safe (Accessed May 4th, 2021)
3. Nardell et Al, “Air Disinfection for Airborne Infection Control with a Focus on COVID-19: Why Germicidal UV is Essential”, Photochemistry and Photobiology, 2021, 97: 493–497
4. Schmarda LK. Der Einfluss des Lichtes auf die Infusionsthierchen. Med Jahrbücher des k. k. Österreichischen Staates. 1845;54:257–70
5. Downes A, Blunt R.P., “Researches on the Effect of Light upon Bacteria and Other Organisms”, Proceedings of the Royal Society of Medicine, 26; 488, 1877
6. Wells WF, Fair MG. “Viability of B. coli exposed to ultra-violet radiation in air.”, Science 1935;82:280-1.
7. Riley RL, Wells WF, Mills CC, Nyka W, McLean RL [1957]. Air hygiene in tuberculosis: quantitative studies of infectivity and control in a pilot ward. Am Rev Tuberc 75(3):420-431.
8. Riley RL, Mills CC, Nyka W, Weinstock N, Storey PB, Sultan LU, Riley MC, Wells WF [1959]. Aerial dissemination of pulmonary tuberculosis: a two-year study of contagion in a tuberculosis ward. Am J Hyg 70:185-196
9. Riley RL, Knight M, Middlebrook G. Ultraviolet susceptibility of BCG and virulent tubercle bacilli. Am Rev Respir Dis 1976;113:413-8.
10. Escombe AR, Moore DAJ, Gilman RH, Navincopa M, Ticona E, Mitchell B, et al. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med 2009;6:e43.
11. M.Mphaphlele, E.A. Nardell, “Controlled Trial of Upper Room Ultraviolet Air Disinfection: A Basis for New Dosing Guidelines”, Am J Respir Crit Care Med Vol 192, Iss 4, pp 477–484, Aug 15, 2015
12. A.H. Malayeri, M.Mohseni, B.Cairns, J.R.Bolton, “Fluence (UV Dose) Required to Achieve Incremental Log Inactivation of Bacteria, Protozoa, Viruses and Algae”, (2016), www.iuvanews.com/stories/pdf/archives/180301_UVSensitivityReview_full.pdf
13. Gates FL. A study of the bactericidal action of ultra violet light: III. The absorption of ultra violet light by bacteria. J Gen Physiol 1930;14:31-42.
14. E.A.Nardell, R.R. Nathavitharana, “Airborne Spread of SARS-CoV-2 and a Potential Role for Air Disinfection”, JAMA. 2020;324(2):141-142
15. [24] L.Marr, S.Miller, K.Prather, C.Haas, W.Bahnfleth, R.Corsi, J.Tang, H.Herrmann, K.Pollitt and J.L.Jimenez, “FAQs on Protecting Yourself from COVID-19 Aerosol Transmission”, https://tinyurl.com/FAQ-aerosols (2020)
16. [25] https://www.medrxiv.org/content/10.1101/2020.10.13.20212233v1
17. WHO website: Coronavirus disease (COVID-19): How is it transmitted? (who.int) (accessed May 14th, 2021)
18. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. doi.org/10.1101/2020.06.15.20132027
19. E.A.Nardell, R.R. Nathavitharana, “Airborne Spread of SARS-CoV-2 and a Potential Role for Air Disinfection”, JAMA. 2020;324(2):141-142
20. L.Marr, S.Miller, K.Prather, C.Haas, W.Bahnfleth, R.Corsi, J.Tang, H.Herrmann, K.Pollitt and J.L.Jimenez, “FAQs on Protecting Yourself from COVID-19 Aerosol Transmission”, https://tinyurl.com/FAQ-aerosols (2020)
21. https://www.medrxiv.org/content/10.1101/2020.10.13.20212233v1
22. WHO guidelines on tuberculosis infection prevention and control (2019 update)
23. https://www.cdc.gov/infectioncontrol/pdf/guidelines/environmental-guidelines-P.pdf
24. Hiatt C. 1964. “Kinetics of the inactivation of viruses.” Bact Rev 28(2):150–163.
25. W.Kowalski, “Ultraviolet Germicidal Irradiation Handbook, UVGI for Air and Surface Disinfection”, 2009
26. ISO 15714, “Method of evaluating the UV dose to airborne microorganisms transiting in-duct ultraviolet germicidal irradiation devices”, Edition 2019-07
27. Innovative Bioanalysis. Efficacy of a wall mounted UV device against aerosolized SARS-CoV-2 https://www.assets.signify.com/is/content/Signify/Assets/philips-lighting/global/20210301-innovative-bioanalysis-report-sars-cov-2.pdf. (accessed May, 28th 2021)
28. C.M.Walker, G.Ko, “Effect of Ultraviolet Germicidal Irradiation on Viral Aerosols”, Environ. Sci. Technol. 2007, 41, 5460-5465
29. N.Storm, L.McKay, S.Downs, R.Johnson, D.Birra, M.de Samber, W.Willaert, G.Cennini, A.Griffiths, “Rapid and complete inactivation of SARS-CoV-2 by ultraviolet-C irradiation”, pre-print version
30. F.M. Collins, “Relative Susceptibility of Acid-Fast and Non-Acid-Fast Bacteria to Ultraviolet Light”, applied microbiology, Mar. 1971, p. 411-413
31. Beggs CB, Avital EJ. 2020. Upper-room ultraviolet air disinfection might help to reduce COVID-19 transmission in buildings: a feasibility study. PeerJ 8:e10196 DOI 10.7717/peerj.10196
32. ASHRAE, Ultraviolet air and surface treatment, Handbook Chapter 62, https://www.ashrae.org/file%20library/technical%20resources/covid-19/i-p_a19_ch62_uvairandsurfacetreatment.pdf (Accessed May 2021)
33. FloEFD computer fluid dynamics simulation tool from Siemens. Simcenter FLOEFD | Siemens Digital Industries Software (Accessed May 2021)